Our skills
Custom water treatment specialist
OUR SKILLS
We master a wide range of treatment and disinfection processes.

Softening

Water treatment
Water softening is a treatment process initially intended to reduce the water hardness (due to the presence of alkaline earth salts : carbonates, sulfates, calcium chlorides and magnesium).
It consists of exchanging calcium and magnesium ions, which are not very soluble and which react with the carbonates in the water to form limestone, against sodium ions which are perfectly soluble in water. We call this operation ion exchange.
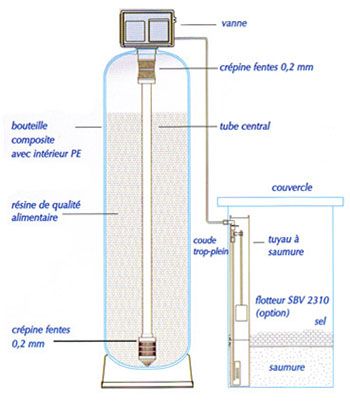
The ionic exchange of the softening is carried out by passing water over a solid support : resin. It is a strong cationic resin carrying sodium ions. It is in the form of porous beads 0.2 to 3 mm in diameter and with a real density slightly greater than that of water. This one has a lot more affinity for calcium and magnesium ions than for sodium ions with which it is originally charged.
When this resin is brought into contact with hard water containing calcium and magnesium ions, the latter bind to the resin by taking the place of sodium ions which were there originally. These sodium ions are released into the water instead of calcium and magnesium ions. The water that a thus percolated from top to bottom on a bed of resin will give up all the calcium and magnesium ions that it contained. Its hardness therefore tends towards zero.
When the resin has given up all the sodium ions with which it was charged, the ion exchange can no longer take place. The resin is said to be “saturated”, the ions Calcium and magnesium can no longer be attached to it, and the water that comes out of the resin bed is as hard as that that goes into it.
It is possible to drive out the calcium ions from the resin and replace them with sodium ions, i.e. to restore the resin to its shape. original. This operation is called “regeneration”. To “regenerate” the saturated resin, it suffices to put it in contact with a solution. very rich in sodium ions.
In practice, we use a concentrated solution of sodium chloride, called “brine”, obtained by dissolving in water. refined salt sold in the form of pellets or granules. As the resin has more affinity for calcium and magnesium than for sodium, to regenerate the resin, it is necessary to use an excess of sodium.


Water hardness :
Hardness of water is the sum of the concentrations of calcium ions (Ca2+) and magnésium (Mg2+) responsible for the formation of limestone. The term “hard water” does not concern the potability of water but only its high content of limestone. The higher the water temperature, the more calcium and magnesium ions are incrustating. The limestone remains, during this process, in the form of small crystals causing problems.
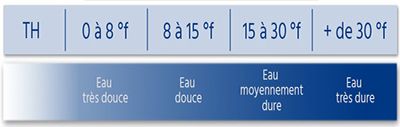
The installation of a softener of water makes it possible to obtain a softened water, unloaded ions of calcium and magnesium. Softened water helps remove limescale and protects your facilities and equipment.
Regeneration :
Once saturated, the resins must be regenerated. In the majority of cases, the regeneration is carried out using sodium chloride (NaCl). A complete regeneration cycle contains the following phases: backwashing, suctioning, rinsing, filling the salt container. The parameters of each of these cycles are adjustable according to the quality of the water and the desired treatment.
Volumetric or chronometric regeneration :
In the context of a volumetric regeneration the softener counts and records the actual water consumption and gives, when the preset volume is exceeded, the signal of the beginning of the regeneration. This can be immediate or pushed back to a predefined time. After regeneration the counter automatically returns to zero and the device is ready again. In the event of a power failure, all data are saved. In the context of a Chronometric or Chronological regeneration the water softener will perform the regenerations at regular intervals of time, every 2 or 3 days for example. Thus, the resin of the softener is regenerated as a function of time, without knowing if it is saturated with magnesium calcium and therefore without knowing if this is really necessary.
Resins :
The resins used meet the FDA (Food and Drug Administration) regulations, are approved by the French Ministry of Health and Public Hygiene and have the ISO9002 certification. The dimensioning of softeners is decisive and must guarantee different criteria :
- optimal passage speed
- choice of resins
- volume of resins
- regeneration frequency
The industrial water softeners that we offer are of high quality and reliability. They find their place in professional uses, to treat large quantities of water (industry, hospitals, communities, etc.). The control valve can be volumetric or chronological, and mechanical or electronic depending on your applications. The choice of the type of valve depends in part on the diameter of the pipe on which the water softener is to be installed.
The softeners proposed by the SFEC have :
- Electronic controls ensuring optimized management of operating cycles.
- Polymer valve body with integrated mixing valve.
- Volume counting by a turbine integrated into the valve body.
- Advanced microprocessor: easily accessible settings on the control panel.
- Three modes of regeneration: immediate volumetric, delayed volumetric or delayed chronometric.
- Double backwash for optimized regeneration and more efficient washing.
- Choice between dry or wet salt pan.
- Cycles and programmable regeneration times.
- Calendar forcing between 1 and 28 days.
- Regeneration against the current or co-current.
- Storing system configuration, operation data and variable reserve in non-volatile memory.
- Save time and hardness for 2 hours during a power failure.
- The salt container is filled with treated water (softened).

Media filtration

Water treatment


Its principle is to cross the water to be treated through a massive consisting of a filter material
(usually a siliceous sand) of a selected particle size. During the passage of water, the fine particles and colloid clusters are retained on the surface of the filtering mass, thus generating a superficial clogging layer which tends to improve the breaking capacity… From a certain loss of load, a phase of retro washing will prevail.
The retro washing phase consists of fluidizing the sand bed by the injection of water and air against the current. The fluidization causes the expansion of the filter media and thus the separation of the particles and the increase of the porosity of the medium. The wash water loaded with these suspended particles is discharged to the sewer. There are three types of sand filtration :
- Rapid sand filters: fast sand filters must be cleaned frequently, by smoothing, which involves reversing the direction of the water.
- Semi-rapid sand filters.
- Slow sand filters.
The first two require pumps and the use of chemicals (flocculation principle). A flocculant that uses a chemical principle to trap suspended solids and particles and form large flakes that will deposit by sedimentation. (Sedimentation means that suspended particles stop moving and settle).
Slow sand filters use biological processes to clean water, and are non-pressurized systems. They can treat water and reduce the presence of microorganisms (bacteria, viruses, microbes…) without the need for chemicals. They do not require electricity to operate.
The operation of these filters without permanent intervention of the operator is possible thanks to the complete automation of the filtration cycles and backwashing. Each filter is equipped with a clogging measurement and a set of automatic valves (water inlet to be filtered, filtered water outlet, washing water inlet, washing air inlet).
Advantages :
This purification process is ecological and often the most economical in emerging countries. It offers the advantage of high efficiency and simple operation. It responds to the needs for water quality improvement while offering the opportunity to involve the community in the management, maintenance and operation of the facilities… Its ability to simultaneously improve the physical, chemical and bacteriological qualities of raw water represents a considerable advantage over other techniques: that of accessing a satisfactory water quality without adding further in other steps of the purification process.
Disadvantages :
However, viruses and bacteria can pass through filters, so the final disinfection step is mandatory. Furthermore, under certain circumstances (climate, raw water quality), a proliferation of certain types of algae can cause a rapid clogging of the filter bed and consequently pose operational problems. Increasing the amount of solids suspended in the raw water, as it is more and more frequently observed, requires cleaning at too frequent intervals. Therefore, if the turbidity exceeds 30 NTU for long periods, pre-treatment by decantation, pre-filtration with horizontal or vertical flow, or other types of pretreatment are essential.

Ultrafiltration

Water treatment
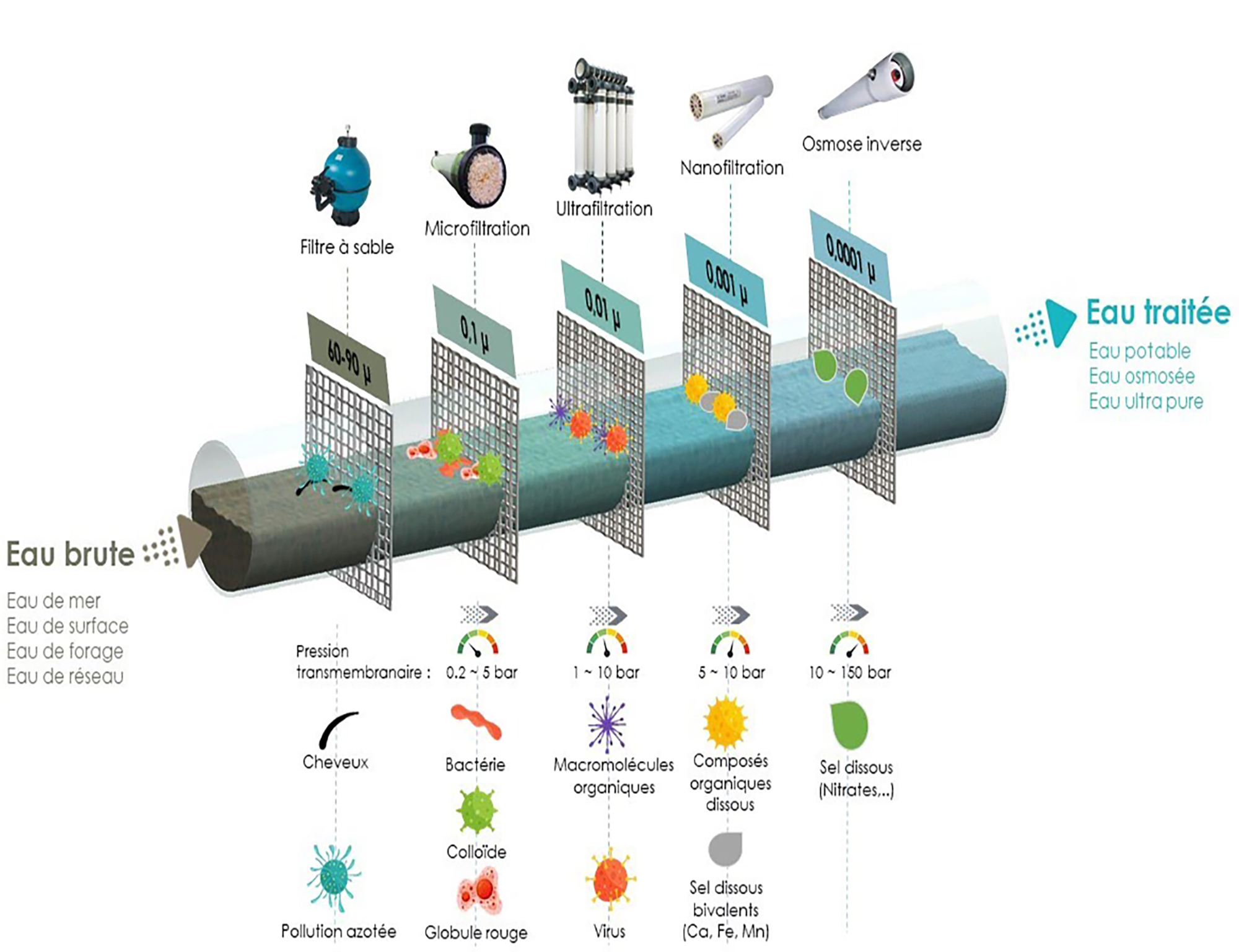
Ultrafiltration is based on the principle of the physical barrier traversed by pressurized water and stopping all elements whose size exceeds a limit value called membrane cutoff threshold..
This technique is used to remove cloudy substances, particles and undesirable microbiological organisms such as germs, bacteria, viruses and parasites present in raw water or when turbidity or microbiological contamination peaks are observed after precipitation.

This process makes it possible to provide pure and crystalline water of uniform quality, without turbidity or pathogens, especially in the treatment of drinking water.
An ultrafiltration module is the basic unit of a membrane filtration system. A module consists of a set of bundles of several thousands of fibers each, protected by grids and secured by 2 resin loaves located at the ends of the module.

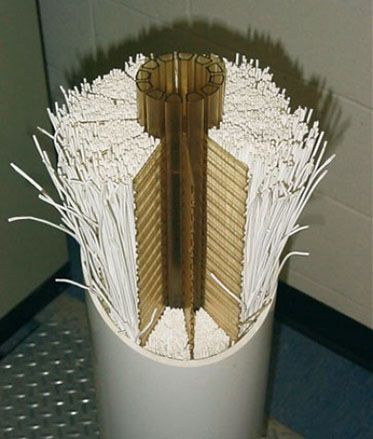
Suspended solids suspended by the membrane will slow down the filtration process. They must be removed periodically. This is the process of backwashing. It is done by sending ultrafiltered water against the current and under pressure through the membrane to remove impurities deposited on its wall.
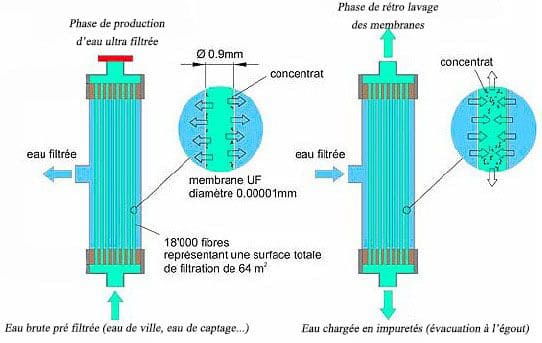
Periodic chemical cleaning may supplement the action of the backwash. These retro chlorinated washings, acid or basic are carried out to thoroughly clean the membrane. Other adjuvants can also be used periodically to automatically regenerate the membrane.
The advantages of ultrafiltration are as follows :
- Remove filter change operations,
- Less sanitization of the process because it is protected against bacteria and micro-organisms,
- The quality of the produced water remains stable,
- Extends the life of any downstream filtration units,
- No products are added to the water,
- The volume of water lost remains very low (at 5% for retro washes),
- Disinfectant product savings,
- Low energy consumption,
- Long life of membranes,
- Reduced maintenance,
- Agreement of the Ministry of Public Health (hollow fiber modules).
Some examples of areas where ultrafiltration is applied :
- Food industry (proteins)
- Dairy industry (milk, cheese)
- Metal industry (oil / water emulsion separation, paint treatment)
- Ultrafiltration can also be applied as pretreatment of water before nano-filtration or reverse osmosis step.

Nanofiltration

Water treatment
Nanofiltration is a technology that produces high quality water through its separation process using filtration through semi-permeable membranes under pressure. Its principle is very similar to that of ultrafiltration, the essential difference being that the nanofiltration membrane has a porosity ten times lower, of the order of 0.001 micrometer.
It makes it possible to retain the micro-pollutants that are the most difficult to eliminate (viruses, bacteria, pesticides, etc.) and almost all the components, be they organic, organic or mineral and whatever their concentration.
The modules used by SFEC are of the tubular or spiral type. Injected under pressure, the water to be treated passes through the membrane and spring filtered through the central tube.
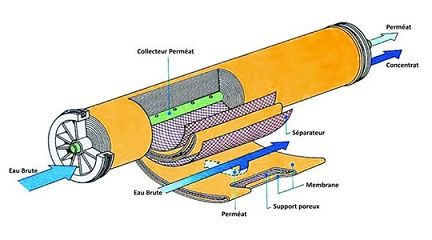

The water molecules that cross the membranes constitute what is called permeate. The permeate is a water that contains only 20 mg/l of sulphates (99% of reduction), 20 mg/l of sodium (92% of reduction) and a hardness of almost zero…
The water molecules and minerals that have not passed through the membranes are guided by the central tube to the second nanofiltration tube to be filtered again and so on to the end of the chain. The remaining part called the concentrate is discharged out of the production line of drinking water (30% of the water sent on the membranes). Nanofiltration makes it possible to retain the micro-pollutants that are the most difficult to eliminate (viruses, bacteria, pesticides, etc…) and almost all the components organic or mineral and whatever their concentration.
Nanofiltration is a safe technique that eliminates all toxic or undesirable substances resulting from human, industrial, agricultural or natural environment activities. In addition, it also significantly reduces the use of chlorine. Its only drawback lies in the fact that the produced water is so pure that it is necessary to remineralize it. Indeed, it stops some ions like Ca2 +.
Today it is mainly used in water purification processes, such as softening, discoloration, and micro-pollutant removal. In industrial processes nano-filtration is used to remove particular components such as coloring agents.
Other applications of nano-filtration :
- Pesticide removal from groundwater
- Elimination of heavy metals from wastewater
- Wastewater recycling in laundries
- Softening
- Elimination of nitrates

Reverse osmosis

Water treatment
Reverse osmosis is a membrane separation technology that uses a membrane system composed of 0.0001 μm pores, through which the water to be treated passes under the effect of a pressure gradient.
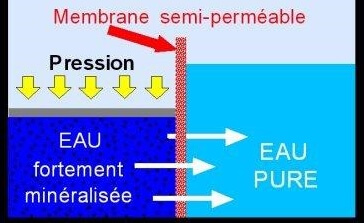
Osmosis membranes release 95 to 99% of elements that are not pure water (salts, minerals, nitrates, phosphates, sulphates, pesticides, herbicides, detergents, heavy metals, chemicals, hormones, pharmaceuticals, radioactivity…). The flow is carried out continuously tangentially to the membrane which limits the accumulation on this membrane of the various species (particles, molecules, ions) retained by the latter.


In the reverse osmosis membrane, the water to be treated is divided into two parts of different concentrations :
- a part which crosses the membrane: it is the permeate (pure osmosis water)
- a part which does not pass through the membrane: it is the concentrate or retentate (dirty water charged with impurities) which contains the molecules or particles retained by the membrane; this “dirty” water is discharged to the sewer.
SFEC reverse osmosis water treatment units require know-how in the selection of equipment (membranes and reverse osmosis modules) and components that must withstand aggressive water (seawater) and at high pressure. Depending on the expected efficiency, it can be used either alone or in combination with other treatment systems. Attention, the reliability of operation and the life of the reverse osmosis systems depend essentially on the quality of the pretreatment implemented in the global sector. It is therefore imperative to ensure that the feed water is properly pretreated to avoid problems of clogging, precipitation, oxidation and pollution of any kind of osmosis membranes…
Pre-treatment is truly a strategic step that must not be neglected because it is the guarantor of the proper functioning of the reverse osmosis system, and especially it is “life insurance” membranes.
The main applications of reverse osmosis concern :
- Desalination of seawater and brackish water,
- Medicine and more specifically the dialysis sector,
- The production of demineralized water for adding water to batteries for example,
- The pharmaceutical industry, or semiconductors, where an osmosis water is essential to the manufacture of the product,
- The agri-food industry, where this process is used to: concentrate sugar from cane sap, beet juice or maple sap, concentrate milk and dairy products to reduce transport costs, extract whey proteins, concentrate the musts in order to increase the final alcohol content of the wines,
- Aquariophilia during partial or complementary water changes,
- Horticulture for watering calcifying plants.

Other processes

Water treatment
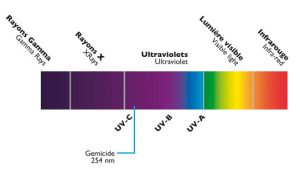
ULTRAVIOLETS
The sun emits an invisible light: the ultraviolet (or UV). This natural phenomenon is reproduced inside the UV treatment devices thanks to powerful lamps, coming from the latest technologies. UV lamps emit UV-C which has a strong bactericidal, virucidal and algicidal power, recognized for a long time.
Two types of lamps exist: low pressure and high pressure lamps.
These emit higher UV-C powers, about 100 to 150 W UV-C but with lower energy yields. The lifetimes of these lamps are approximately 3000 hours for HP type lamps and 8000 hours for BP type lamps.
A UV treatment apparatus consists of one or more lamps placed in quartz sheaths to be thermally insulated from the water. These lamps can be assembled in a cylindrical tube (closed type device) or in a channel (open type device). In both cases the water circulates in the vicinity of the lamps, in thin layers because the UV rays are rapidly absorbed by the water. The quartz sheaths are confined in a reactor which, depending on the operating pressure, is constructed from stainless steel, hot-dip galvanized steel or high-density polyethylene. The assembly is controlled by an electrical cabinet ensuring the lighting of the lamps, their operation, counting the hours of operation and an alarm indicating a possible malfunction.
The energy consumed by the disinfection varies according to the adsorption of the radiation by the water to be treated (turbidity, presence of metals, organic matter …) This energy is generally between 15 and 40 Wh per cubic meter of water. treated water.
The efficiency obtained varies between 90 and 99.99% depending on the exposure time of the water to be treated with radiation. The processing capacity of the devices is very large, from a few liters per hour for a single-lamp device to up to 1,000 cubic meters for larger industrial installations. The investment to be made follows the same evolution.
Ultraviolet type C eradicates germs by greatly disrupting cell metabolism (as they enter their DNA) until they are completely destroyed.

The key elements of effective ultraviolet disinfection are based on : The exposure time (the ratio between the size of the tank and the flow drained by the filtration pump). The energy emitted in micro watts / second / cm² at the furthest distance from the lamp.
By combining the energy emitted by the lamp and the exposure time, the performances will be measured in milijoules (mj).
Advantages and disadvantages :
- + The UV water decontamination system has many advantages. The most interesting is that the disinfection is accompanied by the formation of any reaction product with the organic matter of the water. The use of the apparatus is simple, it is adaptable on a water distribution circuit already in place, its reduced maintenance and its cost of operation is relatively low. And to top it off, UV disinfection is an eco-friendly process that respects the environment.
- – These advantages are thwarted by some major disadvantages. There is no possibility of immediately assessing the effectiveness of the treatment by measuring a residual as in the case of a chemical oxidant. There is no residual effect. The use of UV disinfection is therefore reserved for the disinfection of water whose distribution circuit is short and well maintained. Finally, the proper functioning of the apparatus requires a water of good transmittance, that is to say a turbidity less than 1 NTU.
Application areas :
- Treatment of drinking water. Public and private drinking water supply, hotels, restaurants, hospitals, schools, power stations, military systems, sports centers…
- Agriculture et fish farming. Drinking and general purpose water for farms, dairies, livestock, poultry and fish farming, shellfish…
- Food and beverage industries. Table water, process water (dillutions, rinses), liquid sugar…
- Electronic. Process water for integrated and printed circuits, recycled wash water…
- Chemical, pharmaceutical and cosmetic industries. Production water of high purity, protection against microorganisms developing in tanks, circulation water…
- Photochemistry, air conditioner…
OZONATION
Ozonation is a chemical oxidation treatment. The use of oxidizing chemical reagents for water treatment originally targeted the sterilization of water, or, more accurately, the destruction of pathogenic germs. Ozone has the advantage of allowing complementary actions in the destruction of a large number of micropollutants and in the improvement of tastes, odors and the destruction of colors. Ozone is a molecule with the chemical formula O3. It consists of the chaining of three oxygen atoms, one more than in the case of dioxygen. It is a very unstable gas, which gives it a very important oxidizing capacity. First of all, ozone is created thanks to the ambient air. Indeed, the captured oxygen will pass through an ozone generator where it receives an electric charge. After receiving an electrical charge, some oxygen molecules (O2) will separate to form two independent O atoms. Then these atoms will bind to O2 molecules that have not been split to form ozone molecules (O3).
Ozone is a multifunctional reagent. It destroys toxic compounds such as cyanides and phenols and attacks natural organic dyes (humic acids, tannins, lignins …) and artificial dyes responsible for water coloring. It selectively reacts with organic compounds in water and transforms them into materials that are easier to break down by subsequent biological treatment. In addition, unlike chlorine or chlorine dioxide, ozone does not produce haloforms. It has an effective and fast action but it has little residual action.
Advantages and disadvantages :
- + Ozone decomposes into oxygen, without leaving any by-products in the water.
- + Ozone is produced on site (no transport of toxic products or consumables to be changed regularly)t.
- – La production of ozone consumes energy.
- – The system is quite complex.
- – Some materials are not resistant to ozone.
- – The system requires a significant upfront investment.
There are different types of ozoners :
- The air ozonizer (air dried with a dew point at -50 ° C to -70 ° C).
- Pure oxygen ozonizer with or without oxygen recycling (it does not exceed 100g / m3 of ozone).
- Ozonators also differ in their hourly ozone production capacity. The use of high electrical frequencies significantly improves performance.
There are still two kinds of ozoners :
- Low frequency (50 Hz) ozonators with unit production per hour of approximately 1 to 3 kg of ozone.
- Medium-frequency (150 to 600 Hz) ozonators with a unit output of up to 60 Kg per hour. It is in these ozonators that the ozone is produced and injected into a reactor, where the effluent to be treated is also injected…
And several kinds of reactors :
- Reactors equipped with porous diffusers.
- Reactors equipped with turbines.
- U-tube tube flow reactors equipped with a pump to overcome pressure losses.
Before being injected into the water containing the effluent, the gas containing the ozone can be divided into “micro-bubbles” using various materials :
Porous diffusers arranged in the lower part of the vats or columns. This system has the advantage of not consuming the additional energy and the disadvantage of clogging and aging.
A hydro-injector spraying the gas directly into the driving water at a pressure of 4 to 5 bar. This system has the advantage of a better dissolution rate and the disadvantage of the additional consumption of energy of the driving water pump of the hydro-injector.
The ozone requirement can vary from 2 to 20 g per m3 of water to be treated, depending on the pollutant and its concentration. The electricity consumption is between 20 and 25 Wh per gram of ozone produced. The results obtained speak for themselves: Not only does ozone act on pollutants by improving the transparency of water, it also eliminates iron and manganese, metals that are often responsible for the coloring of water, but it also acts on bacteria. developing in water by eliminating them. Ozone has an important sterilizing power since it has a clear, rapid and radical action on many viruses. Finally, it is a way of eliminating stubborn odors of earth, musty or pharmaceutical without leading to the appearance of tastes as is the case for chlorinated derivatives. Note: it is possible to use coupled techniques (ozone – UV) which have a better efficiency.
Application areas :
Thanks to its excellent qualities of disinfection and oxidation, ozone is enormously used for the treatment of drinking water. Ozone can be used for different purposes in treatment systems, such as for pre-oxidation, intermediate oxidation or final disinfection. Generally, it is recommended to use ozone for pre-oxidation, before an activated carbon filter. After ozonation, these filters can be used to remove the organic matter (important for final disinfection).
ELECTRODEIONISATION
Electrodeionization (EDI) is an electrical purification process using an exchange resin with ion-selective membranes.
It comes in the form of modules. The interior of each EDI module includes a solution containing ions and when a potential electrical is applied to its terminals, cations are attracted to the negatively charged cathode and anions are attracted to the anode positively charged. However, cations can cross the cation permeable membrane, but not into the permeable membrane. to anions. Conversely, anions can cross the anionic membrane, but not the cationic membrane. Thus the water produced in the central part is effectively deionized, since the ions migrate irreversibly towards the electrodes under the electrical influence and focus on adjacent parts.
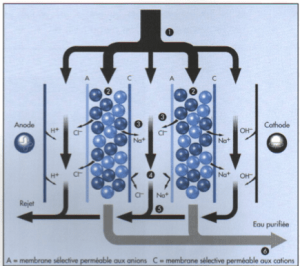
1 : Feed flow.
2 : Dilution compartment.
3 : Concentration compartment.
4 : Ion concentration.
5 : Removal of ions.
A : Anion-permeable selective membrane.
C : Selective cation-permeable membrane.
Thanks to electrodialysis, an electrical potential transports and isolates charged aqueous species. Electric current is charged for continuously regenerate the resin, eliminating the need for periodic regeneration.
Unlike traditional ion exchange in which the resins are used up and must be either discarded or chemically regenerated, the EDI process uses an electric current allowing continuous regeneration of the resins.
Placed downstream of reverse osmosis installations, the electrodeionization process makes it possible to re-treat the osmosis water and thus obtain ultra pure water with very low conductivity and silicic acid content.




Advantages and disadvantages :
As a replacement for traditional ion exchange processes, EDI provides advantages both in terms of energy and expenses for high purity water treatment. By eliminating the need for periodic regeneration of the ion exchange resin, environmental benefits are also achieved by avoiding the handling and processing of acidic and caustic chemicals brought to the site.
Some advantages of EDI over conventional ion exchange systems are :
- + Simple and continuous operation
- + Chemicals for regeneration are completely eliminated
- + Actual cost of operation and maintenance
- + Low energy consumed
- + Non-polluting, safe and reliable
- + It requires very few automatic valves or complex instructions for execution and needing supervision by an operator
- + It requires very little space
- + It produces very pure water at a constant flow
- + It allows the complete removal of dissolved inorganic particles
- + In combination with reverse osmosis pre-treatment, it removes over 99.9% of ions from water
EDI cannot be used for water with a hardness greater than 1, since the calcium carbonate would create deposits limiting the operation.
It requires a purification pre-treatment.
Carbon dioxide will pass freely through the reverse osmosis membrane, breaking down and increasing the conductivity of the water. All the Ion species formed by the carbon dioxide gas will lower the resistivity of the output water produced by EDI. CO2 management in water is typically done in one or two ways: the pH of the water can be adjusted to allow reverse osmosis membranes to release ionic species or carbon dioxide can be removed from water using an entraining gas.
Application areas :
EDI is useful for any application that requires continuous and economical removal of impurities from water without the use of dangerous chemicals such as :
- Reuse of residual water in the food industry
- Production of chemicals
- Biotechnology
- Electronics
- Cosmetic
- Laboratories
- Pharmaceutical industry
- Boiler feed water
- Reduction of ionizable SiO2 and TOC (Total Organic Carbon)
Treatment processes
Useful links
Newsletter
Subscribe to our newsletter to get updated information, news, information or promotions.